About Us

Who We Are
From its beginnings as a consulting firm to its position today as a global clinical research organization (CRO), Aesculape CRO offer a full range of consulting, development and commercialisation services from a global network of our offices.
Aesculape’s objectives are shaped by its commitment to consistent quality and execution, exceptional customized approach and service to speed the overall execution of clinical trials.
Aesculape CRO is providing the highest level of quality in the execution of clinical trials while offering full service capabilities from Phase I to IV in various geographies around the world, with a network of clinical trial management offices throughout Eastern, Central and Western Europe and South East Asia.
Our dedicated staff with scientific and/or medical backgrounds can cover all areas of clinical research, ranging from clinical operations (site selection and feasibility, submissions to regulatory authorities, monitoring) to medical writing, pharmacovigilance and marketing authorization.
In summary, Aesculape CRO has the know-how, experience, infrastructure and capacity to provide our clients with the requested services to a high degree of satisfaction.
Our Philosophy & Values
Our Work Is Heavily Shaped by Our Philosophy & Core Values
01.
Objective
Our objective is to convey operational skill & knowledge through harmonized productivity for project goal achievement.
02.
Scientific Approach
In clinical trial business challenges, Aesculape CRO adopts a scientific approach to assess risk management and objectively increase productivity for success.
03.
Mission
Deliver quality of research & development (R&D) through strategic cost-effective plan & expertise knowledge and services.
04.
Values
- Ethical study conduction
- Integrity
- Project transparency
- Reduced trial times
- Reaching the milestones fast and in a cost-effective way

Our Expertise
We focus our priorities and actions on the factors that are critical to our clients – reducing time to market, reducing cost and increasing quality.
Our global team of experts has extensive experience in a wide range of therapeutic areas and can cover all areas of clinical research, ranging from clinical operations (site selection and feasibility, submissions to regulatory authorities, monitoring) to medical writing, pharmacovigilance and marketing authorization.
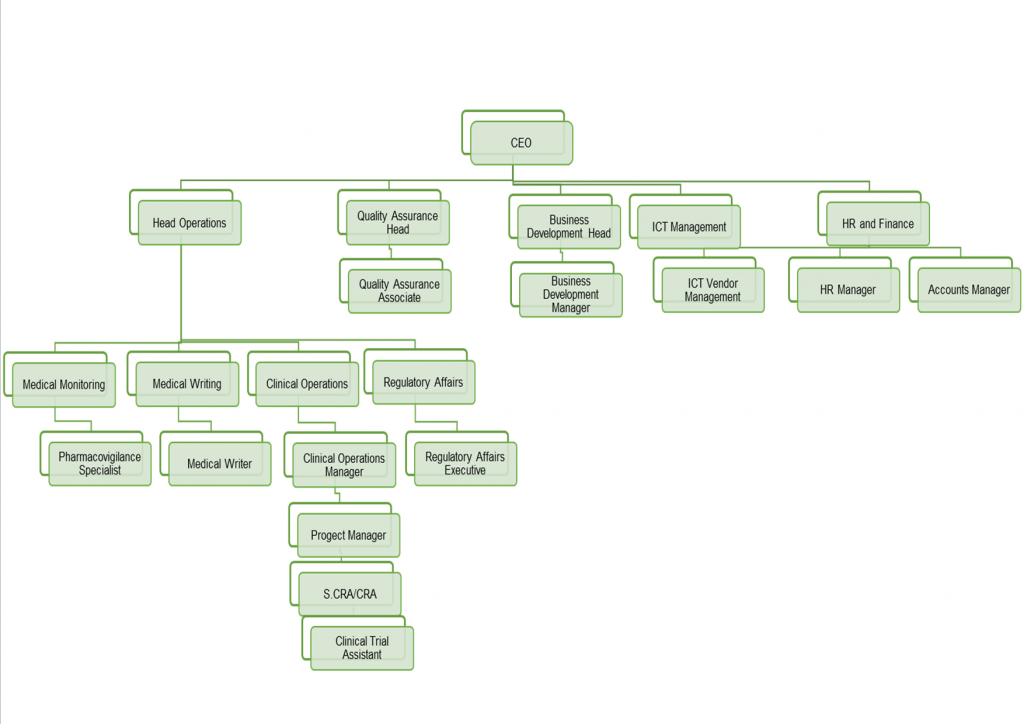
Organisational Structure
Our Organisational Structure

CEO Message
Aesculape CRO Asia Pte Ltd is a Singapore-based Clinical Research Organisation, aimed to provide full clinical trial services for pharmaceutical, medical devices, biotech & nutraceutical companies wanting to register and commercialize their products in Europe, the US, and Asia.
We are active in South and Southeast Asia as well as in West, Central, and Eastern Europe, but have trusted partners in other parts of the world.
Our company philosophy is “TO BE AN ENGINE OF MEDICAL INNOVATION” and to achieve this we stand for productivity and efficiency at all levels, through the use of advanced technology. Since the Clinical Research industry is a competitive and challenging business environment, Aesculape CRO is aware of the importance of teaching and training all its staff members as well as the professionals it is engaging with. For this, we have recently created the Aesculape Academy in collaboration with Kings College London and with the Faculty of Pharmaceutical Medicine of the Royal College of Physicians of the UK. Herein we have opted for a blended learning system accessible to all interested in learning more about Clinical Research and Pharmaceutical Medicine.
Our aim is to ensure that thanks to the professionals of Aesculape, you will be able to achieve your goals and, most importantly, shorten your time to registration. Our team members are trained to listen to your specific needs and go out of their way to look for solutions, hereby helped by their excellent connections in Academia and their good knowledge of regulatory pathways.
Let us be part of your journey and in this ensure better health and quality of life for millions of patients!
Sincerely, CEO. Jean-Paul Deslypere
Locations
Global Presence Across Europe & Asia
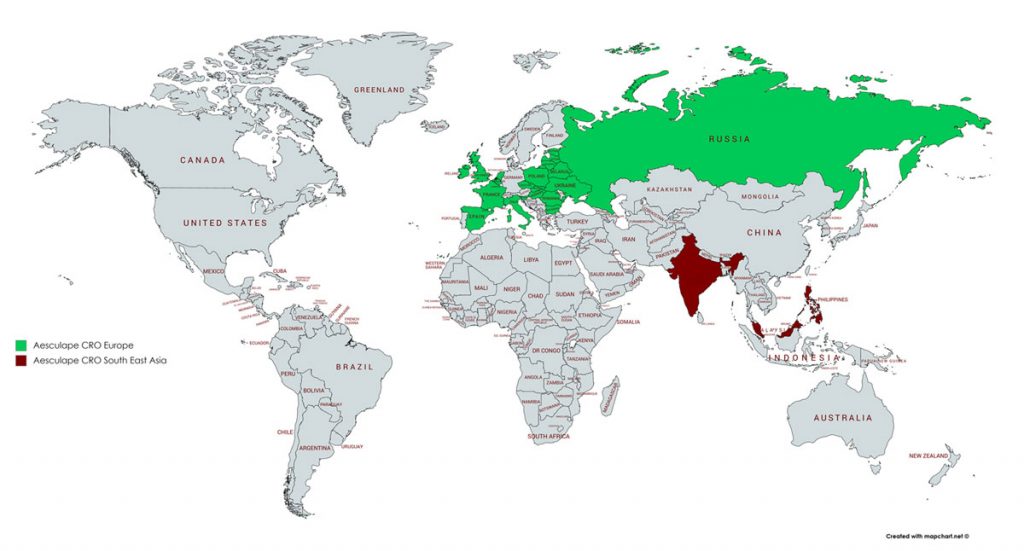
With offices in 9 countries and more than 30 professionals worldwide, Aesculape CRO applies therapeutic experience, expertise and an excellent organization to help customers and partners bend the cost and time curve of drug development.
Our regional team members dedicated to deliver professional services in various languages including, Malay, Thai, Tagalog, Hindi, Urdu, Chinese, French, Italian, Dutch, Spanish, German, Polish, Russian and all staff members speak English.
Call Today
+65 9826 2598 +32 492 73 59 31
Drop us an email at
info@aesculape.com
